
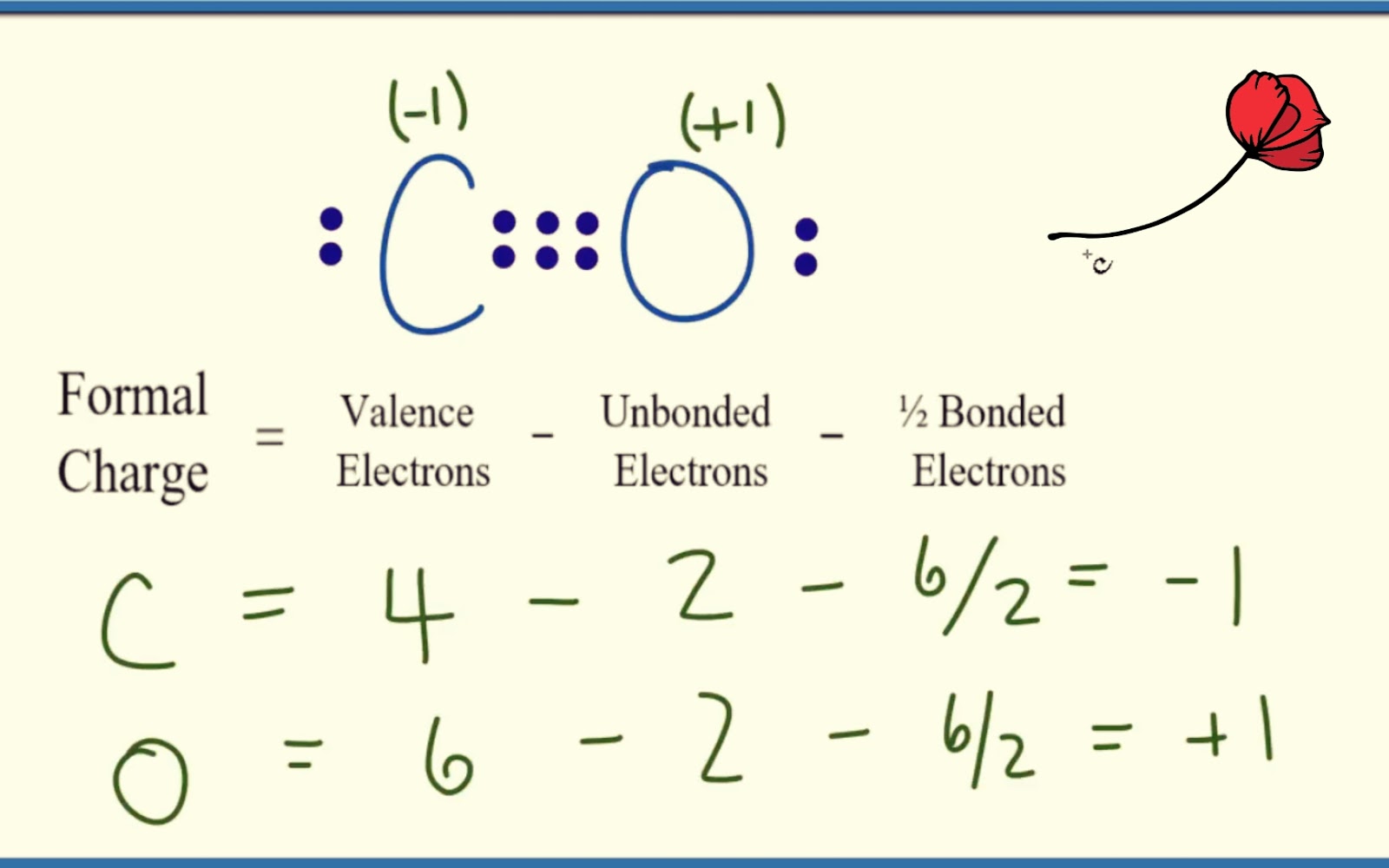
Usually, negative charge should be on the Oxygen is more electronegative than Carbon atom. What is the special thing of CO lewis structure considering the charges? According to the most stable lewis structure of CO, there is a triple bond between Carbon atom and Oxygen atom. Also, there are four bonds around the carbon atom in CO 2. In the lewis structure of CO, there is a lone pair on Carbon atom. What are the differences of lewis structures of CO and CO 2? But, there will be +1 charge on oxygen atomĪsk your chemistry questions and find the answers Then, there will be a triple bond between carbon and oxygen atoms. So, we need to convert one more lone pair However above structure is still unstable because octal is not still completed in carbon atom. Therefore, above structure is notĪnd need to convert a lone pair on oxygen atoms to a bond as below. Also octal is not completed in carbon atom. Marking of charges on atoms is a compulsory step to decide the stability of a molecule.Ĭheck the stability and minimize charges on atoms by converting lone pairs to bondsīecause, there are charges on carbon and oxygen atoms. There are charges on carbon and oxygen atoms to mark in the above structure.

However those all steps are mentioned and explained in detail in this tutorial for your knowledge. Whether CO molecule seems as a simple molecule, there are important things to consider drawing the lewis structure. Number of steps can be changed according the complexity of the molecule or ion. There are guidelines (several steps) to draw a lewis structure. Steps of drawing lewis structure of CO molecule

Somehow trying to achieve the octet rule.
#Co carbon monoxide lewis structure how to#
Now, we will learn how to draw the carbon monoxide lewis structure step by step. of CO is a triple bond be- tween the carbon and the oxygen and one lone pair on each atom. However, carbon monoxide is easily oxidized to You should never try to get exposed to this carbon monoxide gas. In next sections, we will draw CO lewis structure step by step.Ĭarbon monoxide is a very toxic gaseous molecule at room temperature and formed due to incomplete combustion of products However, oxygen atoms has a +1 charge andĬarbon atom has a -1 charge. In the lewis structure of carbon monoxide, both atoms have eight electrons in their valence shells. Carbon monoxide has one sigma bond and two pi bonds.Lewis structure of Carbon monoxide (CO) molecule The first bond between a pair of atoms is sometimes called a sigma bond. The additional bond between a pair of atoms is often called a pi bond (pronounce "pie" bond). Comparison of bond length between carbon monoxide (1.13 Ångstroms) and carbon dioxide (1.16 Ångstroms). \): A comparison of experimentally determined bond distances.


 0 kommentar(er)
0 kommentar(er)
